Fill Out a Valid Medication Error Template
Guide to Writing Medication Error
Filling out the Medication Error form is a crucial step in ensuring patient safety and improving pharmacy practices. After completing the form, it will be submitted to the appropriate authorities for review and action. This process helps to identify issues and implement necessary changes.
- Begin by entering the Incident Report # at the top of the form.
- Fill in the Patient Information section, including the patient's name, address, phone number, sex, date of birth, prescription number, and PHIN.
- Record the Error Date and the Discovery Date along with the hour, date, month, and year.
- In the Drug ordered section, clearly state the drug, dose, form, route, and directions for use.
- Choose the appropriate Type of Incident that applies to the patient receiving the drug and mark the relevant box.
- If applicable, select the Type of Incident or Discrepancy for the patient who did not receive the drug and provide specific details.
- In the Incident/Discrepancy Description section, write a detailed account of the facts as known at the time of discovery. Attach additional details if necessary.
- Sign and date the form in the Signature of Pharmacist section.
- Complete the Contributing Factors section by marking any relevant factors that contributed to the incident.
- Fill in the Notification section, indicating whether the patient and physician were notified, along with the respective dates and times.
- Select the appropriate Severity level based on the incident's impact on the patient.
- In the Outcome of Investigation Follow-Up section, indicate the problem identification and actions taken.
- Document the Resolution of Problem section, including signatures and dates from both the pharmacist filling out the form and the pharmacy manager.
Document Breakdown
| Fact Name | Description |
|---|---|
| Purpose | This form is used to report all medication incidents and discrepancies. |
| Initiation | The pharmacist who discovers the error is responsible for initiating the report. |
| Notification Requirement | All medication incidents affecting patient health or safety must be reported to the physician and pharmacy manager. |
| Patient Information | The form requires detailed patient information, including name, address, phone number, sex, and date of birth. |
| Error Date | The date of the medication error must be clearly recorded. |
| Incident Types | Multiple types of incidents can be reported, including incorrect dose, incorrect drug, and allergic reactions. |
| Contributing Factors | Pharmacists must identify contributing factors such as improper patient identification or misinterpreted drug orders. |
| Severity Levels | The form includes a section to classify the severity of the incident, ranging from none to requiring immediate medical intervention. |
| Outcome Follow-Up | Follow-up actions are required to address problems identified during the investigation. |
| State-Specific Laws | Each state may have specific laws governing medication error reporting; refer to local regulations for details. |
FAQ
What is the purpose of the Medication Error form?
The Medication Error form is designed to report any incidents or discrepancies related to medication. This includes both errors that have affected a patient and those that have not yet reached the patient. It serves as a tool for pharmacists to document and communicate issues that could impact patient safety.
Who is responsible for initiating the report?
The pharmacist who discovers the medication error is responsible for initiating the report. This ensures that the individual most familiar with the incident provides accurate details and context for the situation.
When should a physician and pharmacy manager be notified?
Notification should occur for all medication incidents that could potentially affect a patient's health or safety. This is crucial for ensuring that appropriate actions are taken to address the situation and prevent future occurrences.
What types of incidents can be reported?
There are various types of incidents that can be reported on the form, including:
- Incorrect Dose
- Incorrect Dosage Form
- Incorrect Drug
- Incorrect Generic Selection
- Incorrect Patient
- Incorrect Strength
- Outdated Product
- Allergic Drug Reaction
- Incorrect Label/Directions
- Drug Unavailable/Omission
- Drug-drug Interaction
- Other (specify)
What information is required on the form?
The form requires detailed patient information, including the patient's name, address, phone number, sex, date of birth, prescription number, and the pharmacist's information. Additionally, it asks for the date of the error, the type of incident, and a description of the incident or discrepancy.
What should be included in the incident description?
The incident description should state the facts as known at the time of discovery. It may include specific details about the error and any contributing factors. If necessary, additional details can be attached to the form for clarity.
How are contributing factors documented?
Contributing factors are to be completed by the pharmacist responsible for the incident. This section includes options such as improper patient identification, misreading drug orders, and lack of patient counseling. The pharmacist can also specify any other factors that contributed to the error.
What is the severity scale used in the form?
The severity of the incident is categorized into several levels, including:
- None
- No change in patient’s condition: no medical intervention
- Minor required
- Major
- Produces a temporary systemic or localized response: does not cause ongoing complications
- Requires immediate medical intervention
What happens after the form is submitted?
After the form is submitted, an investigation follows to identify the problem and implement solutions. This may involve providing education, changing policies or procedures, or increasing awareness among staff. The resolution of the problem will also be documented on the form.
Is there a need for follow-up after reporting an incident?
Yes, follow-up is essential. The outcome of the investigation should be documented, including actions taken to resolve the issue and prevent recurrence. This helps to ensure accountability and improve medication safety practices within the pharmacy.
Fill out Other Forms
Parent Permission Form - Filling out this form is an essential step for participation.
To facilitate a smooth transaction, it is advisable to use a well-crafted Bill of Sale form, such as one provided by Formaid Org, which ensures that all necessary information is captured and both parties are protected throughout the sale process.
What Happens to a Jointly Owned Property If One Owner Dies in California - The document serves to clarify the status of property ownership after a joint tenant's death.
6 Team Single Elimination Bracket With Consolation - Helps maintain focus on the significance of each game in the consolation bracket.
Medication Error Example
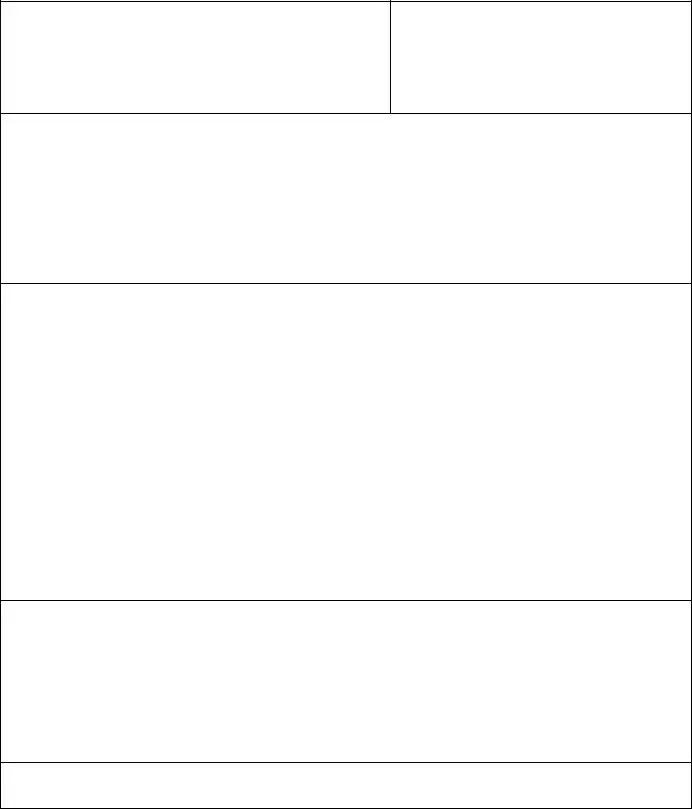
MEDICATION INCIDENT AND DISCREPANCY REPORT FORM |
Incident Report #: |
MEDICATION INCIDENT AND DISCREPANCY REPORT
1.Use for all medication incidents. Medication discrepancies can be reported at pharmacist’s discretion.
2.The pharmacist discovering the error initiates the report
3.Notify physician and pharmacy manager of all MEDICATION INCIDENTS that could affect the health or safety of a patient
PATIENT INFORMATION
Name:____________________________________
Address:__________________________________
Phone:____________________________________
Sex: _____ DOB:_________________________
Rx #:_____________________________________
PHIN_____________________________________
Error Date: |
______________________________ |
Pharmacist initiating |
|
|||
|
Hour |
Date |
Month |
Year |
report: |
______________________ |
Discovery Date: |
______________________________ |
|
|
|||
|
Hour |
Date |
Month |
Year |
|
|
Drug ordered: |
|
|
|
|
|
|
(State: drug/dose/form/route/directions for use) |
|
|
|
|||
Medication Incident: an erroneous medication commission or omission that has been subjected upon a patient.
Medication Discrepancy: an erroneous medication commission or omission that has not been released for the patient.
TYPE OF INCIDENT– Patient received drug: |
|
|
|
||
|
Incorrect Dose |
|
Incorrect Dosage Form |
|
Incorrect Drug |
|
Incorrect Generic Selection |
|
Incorrect Patient |
|
Incorrect Strength |
|
Outdated Product |
|
Allergic Drug Reaction |
|
Incorrect Label/Directions |
|
Drug Unavailable/Omission |
|
|
Other ________________ |
|
______________________________________________________________________________________________
______________________________________________________________________________________________
______________________________________________________________________________________________
TYPE OF INCIDENT OR DISCREPANCY – Patient did not receive drug:
Prescribing (specify) _______________________________________________________________________
Dispensing (specify) _______________________________________________________________________
Documentation (specify) ____________________________________________________________________
Other (specify) ____________________________________________________________________________
INCIDENT/DISCREPANCY DESCRIPTION
State facts as known at time of discovery. Additional details about the error by the pharmacist involved may be attached to this document.
________________________________________________________________________________________________
________________________________________________________________________________________________
________________________________________________________________________________________________
________________________________________________________________________________________________
________________________________________________________________________________________________
________________________________________________________________________________________________
DATE: |
______________________________ |
________________________________ |
|
Hour Date Month Year |
Signature of Pharmacist: |
Page 1 of 2

CONTRIBUTING FACTORS
(To be completed by pharmacist responsible)
|
Improper patient identification |
Misread/misinterpreted drug order (include verbal orders) |
|
|
Incorrect transcription |
|
Drug unavailable |
Lack of patient counselling |
|
Other |
|
|
DATE: |
______________________________ |
__________________ |
|
|
||||
|
|
Hour Date Month Year |
Signature |
|
|
|
|||
|
NOTIFICATION – Complete the following information according to Standards of Practice. |
||||||||
1. |
Patient notified: |
|
|
|
|
|
|
|
|
|
|
|
___________________________ |
|
|||||
|
|
|
Hour |
Date |
Month |
Year |
|||
2. |
Physician notified: ____ |
______________________________ |
|
||||||
|
|
Yes/No |
Hour |
Date |
Month |
Year |
|||
|
|
|
|
|
|
|
|
|
|
|
SEVERITY |
|
|
|
|
|
|
|
|
|
|
None |
|
No change in patient’s condition: no medical intervention |
|||||
|
|
Minor |
|
|
|
required |
|
|
|
|
|
Major |
|
Produces a temporary systemic or localized response: does |
|||||
|
|
|
|
|
|
not cause ongoing complications |
|||
|
|
|
|
Requires immediate medical intervention |
|||||
|
OUTCOME OF INVESTIGATION |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
Problem Identification |
|
|
|
Action |
|
|
|
|
|
|
Lack of knowledge |
|
|
Education provided |
||||
|
|
Performance problem |
|
|
Policy/procedure changed |
||||
|
|
Administration problem |
|
|
System changed |
|
|
||
|
|
Other |
|
|
Individual awareness |
||||
|
|
|
|
|
Group awareness |
||||
|
|
|
|
|
Other |
|
|
|
|
|
|
|
|
|
|||||
|
RESOLUTION OF PROBLEM THAT RESULTED IN THE ERROR BEING MADE: |
||||||||
|
|
|
|
|
|
|
|
||
|
Signature: |
Date: |
Signature: |
Date: |
|||||
|
(Pharmacist filling out the form) |
|
|
|
(Pharmacy Manager) |
||||
PHARMACY USE ONLY
Page 2 of 2
