Fill Out a Valid Drug Screen Template
Guide to Writing Drug Screen
Filling out the Drug Screen form is an important step in the testing process. It ensures that all necessary information is accurately recorded and that the chain of custody is maintained. Follow the steps below to complete the form correctly.
- Start by entering the Employer Name, Address, and I.D. No. in the designated section.
- Provide the MRO Name, Address, Phone, and Fax No. in the next section.
- Fill in the Donor SSN or Employee I.D. No. as required.
- Specify the Testing Authority by checking the appropriate box (HHS, NRC, DOT) and indicating the DOT Agency if applicable.
- Indicate the Reason for Test by selecting from the options provided or writing in another reason.
- List the Drug Tests to be Performed by checking the appropriate boxes or specifying other tests if necessary.
- Complete the Collection Site Name, Code, Address, Phone No., City, State, and Zip in the respective fields.
- In the next section, the Collector should read the specimen temperature within 4 minutes and indicate if it is between 90° and 100° F. Note any remarks as needed.
- Specify the type of Collection (Split, Single, None Provided) and make remarks if necessary.
- The Collector must affix bottle seals to the specimen bottles, date the seals, and have the donor initial them.
- Ensure the donor completes STEP 5 on Copy 2 (MRO Copy).
- For the Chain of Custody, the collector must certify that the specimen was collected, labeled, sealed, and released according to Federal requirements. Include the signature, date, and time of collection.
- Document the name of the Delivery Service used for transporting the specimen.
- Upon receipt at the lab, check if the Bottle Seal is intact and note any remarks if it is not.
- Complete the Primary Specimen Report by indicating if the results are negative, positive, or rejected for testing.
- Finally, the certifying scientist must sign and date the form to verify that the specimen was handled and analyzed according to Federal requirements.
Document Breakdown
| Fact Name | Description |
|---|---|
| Form Title | This is the Federal Drug Testing Custody and Control Form. |
| Contact Number | The form includes a contact number: 800-877-7484. |
| Specimen ID | Each specimen is assigned a unique Specimen ID number. |
| Employer Information | Employers must provide their name, address, and ID number. |
| MRO Details | Medical Review Officer (MRO) information is required, including name, address, phone, and fax number. |
| Testing Authority | Testing can be authorized by HHS, NRC, or DOT, with specific DOT agencies listed. |
| Reason for Testing | Multiple reasons for testing are provided, including pre-employment and random testing. |
| Drug Tests | Tests may include THC, COC, PCP, OPI, AMP, or combinations thereof. |
| Chain of Custody | The form ensures a chain of custody from collection to testing, which must be documented. |
| Certification | Certifying scientists must sign and date the form, confirming compliance with federal requirements. |
FAQ
What is the purpose of the Drug Screen form?
The Drug Screen form is used to document the collection and testing of urine specimens for drug use. It ensures compliance with federal regulations and helps maintain a safe workplace by identifying individuals who may be using illegal substances. Employers use this form for various reasons, including pre-employment screening, random testing, and follow-up tests after an incident.
Who is responsible for completing the Drug Screen form?
The form is typically completed by the collector or an employer representative. They are responsible for accurately documenting all required information, including donor identification, testing authority, and the reason for the test. Proper completion is crucial to ensure the integrity of the testing process.
What information is required on the form?
The form requires several key pieces of information:
- Employer name and address
- Medical Review Officer (MRO) contact details
- Donor's Social Security Number or Employee ID
- Testing authority and reason for the test
- Types of drug tests to be performed
- Collection site details
Each of these elements plays a vital role in ensuring the test is valid and traceable.
What happens during the specimen collection process?
During the collection process, the collector will verify the specimen temperature within four minutes to ensure it falls within the acceptable range of 90° to 100° F. The collector will then label and seal the specimen bottles, ensuring the donor initials the seals. Observations and any remarks about the collection are documented on the form.
How is the chain of custody maintained?
Chain of custody is a critical aspect of the drug testing process. It involves documenting every person who handles the specimen from collection to testing. The collector certifies that the specimen was collected and sealed properly. This information is crucial for maintaining the integrity of the test results and ensuring that they are legally defensible.
What does it mean if a test result is reported as "positive" or "negative"?
A "negative" result indicates that no illegal substances were detected in the specimen. Conversely, a "positive" result means that one or more substances were found. The specific substances detected will be listed on the form, providing clarity on the findings. If a result is reported as "dilute," "adulterated," or "invalid," further investigation may be necessary.
What should I do if I have questions about my test results?
If you have questions or concerns about your test results, it’s important to reach out to your employer or the Medical Review Officer (MRO) listed on the form. They can provide clarification and guidance on the next steps, especially if a positive result was reported. Open communication is key to addressing any misunderstandings or issues.
Fill out Other Forms
What Does Qdro Stand for - Only original court-certified orders will qualify for compliance with the QDRO form requirements.
Physical Exam Form for Healthcare Workers - Report any communicable diseases and necessary precautions to prevent spread.
Understanding the significance of a Living Will is crucial; it is a vital document that grants individuals the authority to specify their medical treatment preferences. For a thorough overview, explore the comprehensive details about the Living Will form available at the following link: essential Living Will form information.
Lien Release Requirements by State - The form allows attachment of pertinent documents to strengthen the claim.
Drug Screen Example
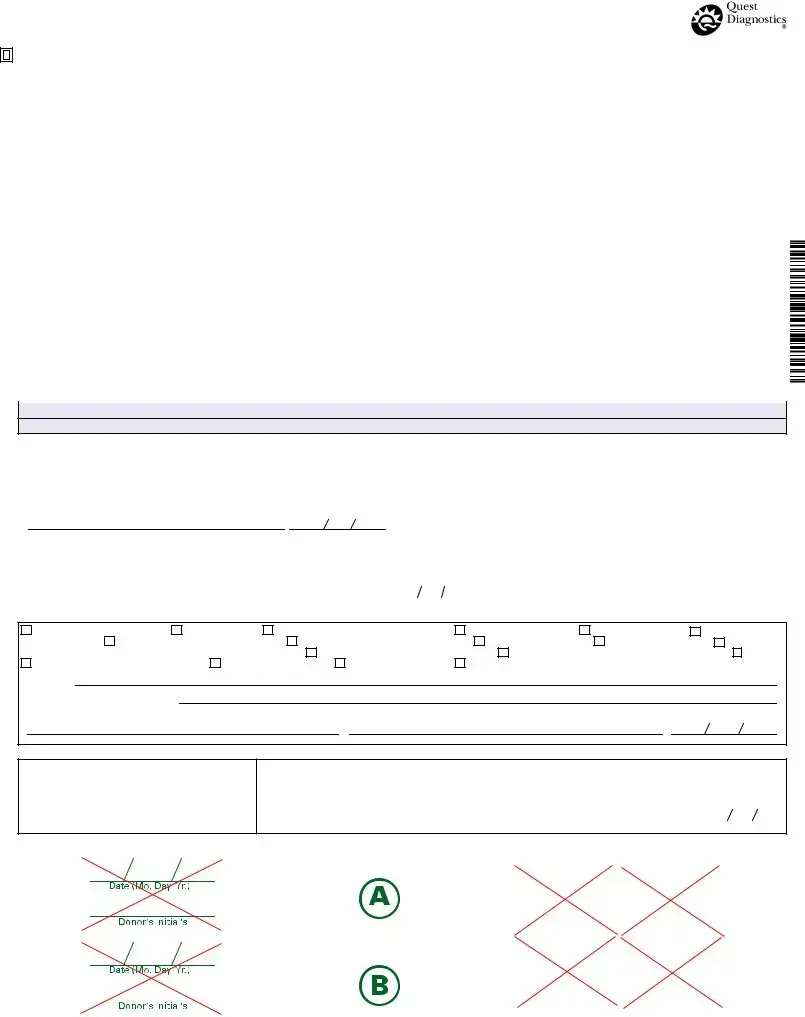
FEDERAL DRUG TESTING CUSTODY AND CONTROL FORM
SPECIMEN ID NO. |
|
STEP 1: COMPLETED BY COLLECTOR OR EMPLOYER REPRESENTATIVE |
LAB ACCESSION NO. |
Quest, Quest Diagnostics, the associated logo and all associated Quest Diagnostics marks are the trademarks of Quest Diagnostics Incorporated. © Quest Diagnostics Incorporated. All rights reserved.
A. Employer Name, Address, I.D. No. |
|
|
B. MRO Name, Address, Phone and Fax No. |
||||||||||
|
|
|
|
|
|
|
|
|
|||||
C. Donor SSN or Employee I.D. No. _______________________________________________________________ |
|
|
|
|
|||||||||
D. SpecifyTesting Authority: HHS |
NRC |
DOT – Specify DOT Agency: FMCSA |
FAA |
FRA FTA PHMSA USCG |
|||||||||
E. Reason forTest: |
Random |
Reasonable Suspicion Cause Post Accident |
Return to Duty |
|
|||||||||
F. DrugTests to be Performed: |
THC, COC, PCP, OPI, AMP |
THC & COC Only |
Other (specify) ________________________________________________ |
||||||||||
G. Collection Site Name: |
|
|
|
|
|
Collection Site Code: |
|
|
|
|
|||
Address: |
|
|
|
|
|
|
Collector Phone No.: |
|
|
||||
City, State and Zip: |
|
|
|
|
|
Collector Fax No.: |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STEP 2: COMPLETED BY COLLECTOR (make remarks when appropriate) Collector reads specimen temperature within 4 minutes.
Temperature between 90° and 100° F? Yes No, Enter Remark |
Collection: Split Single None Provided, Enter Remark |
Observed, (Enter Remark) |
REMARKS
STEP 3: Collector affixes bottle seal(s) to bottle(s). Collector dates seal(s). Donor initials seal(s). Donor completes STEP 5 on Copy 2 (MRO Copy)
STEP 4: CHAIN OF CUSTODY - INITIATED BY COLLECTOR AND COMPLETED BY TEST FACILITY
|
I certify that the specimen given to me by the donor identified in the certification section on Copy 2 of this form was |
|
SPECIMEN BOTTLE(S) RELEASED TO: |
|||||||
|
collected, labeled, sealed, and released to the Delivery Service noted in accordance with applicable Federal requirements. |
Quest Diagnostics Courier |
||||||||
|
|
X |
|
|
|
|
|
FedEx |
||
|
|
Signature of Collector |
|
|
|
|
|
Other |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AM |
|
|
|
|
|
|
|
|
|
|
PM |
|
|
|
|
|
|
(Print) Collector's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|
Time of Collection |
|
|
Name of Delivery Service |
||
RECEIVED AT LAB OR IITF: |
|
|
|
|
|
Primary Specimen |
SPECIMEN BOTTLE(S) RELEASED TO: |
|||
|
X |
|
|
|
|
|
Bottle Seal Intact |
|
||
|
|
|
|
|
|
Yes No |
|
|||
|
|
Signature of Accessioner |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If No, Enter remarks |
|
|
|
|
|
|
|
|
|
|
in Step 5A. |
|
|
|
|
(Print) Accessioner’s Name (First, MI, Last) |
|
|
|
Date (Mo./Day/Yr.) |
|
|||
STEP 5A: PRIMARY SPECIMEN REPORT - COMPLETED BY TEST FACILITY
NEGATIVE |
POSITIVE for: |
Marijuana Metabolite ( |
6- Acetylmorphine |
Methamphetamine |
MDMA |
|
DILUTE |
|
|
Cocaine Metabolite (BZE) |
Morphine |
Amphetamine |
MDA |
|
|
|
PCP |
Codeine |
|
MDEA |
REJECTED FOR TESTING |
ADULTERATED |
SUBSTITUTED |
INVALID RESULT |
|
|
|
REMARKS:
Test Facility (if different from above):
I certify that the specimen identified on this form was examined upon receipt, handled using chain of custody procedures, analyzed, and reported in accordance with applicable Federal requirements.
X
Signature of Certifying Scientist |
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
STEP 5b: COMPLETED BY SPLIT TESTING LABORATORY
RECONFIRMED FAILED TO RECONFIRM - REASON ____________________________________________
___________________________________________ |
I certify that the split specimen identified on this form was examined upon receipt, handled using chain of custody |
||||||||||||||||||||||||||||
procedures, analyzed and reported in accordance with applicable Federal requirements. |
|
|
|
|
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Name |
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
___________________________________________ |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Signature of Certifying Scientist |
|
|
|
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Address |
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OMB No.
PRESS HARD - YOU ARE MAKING MULTIPLE COPIES
